Coronavirus: Pfizer seeks approval to administer BioNTech jab to under 12s, but Hong Kong doctors are cautious
- The pharmaceutical giant is seeking FDA authorisation to offer the jabs to younger kids after successful trials with children aged five to 11
- Some medical professionals in Hong Kong want to wait, as kids are at low risk of contracting Covid in the city
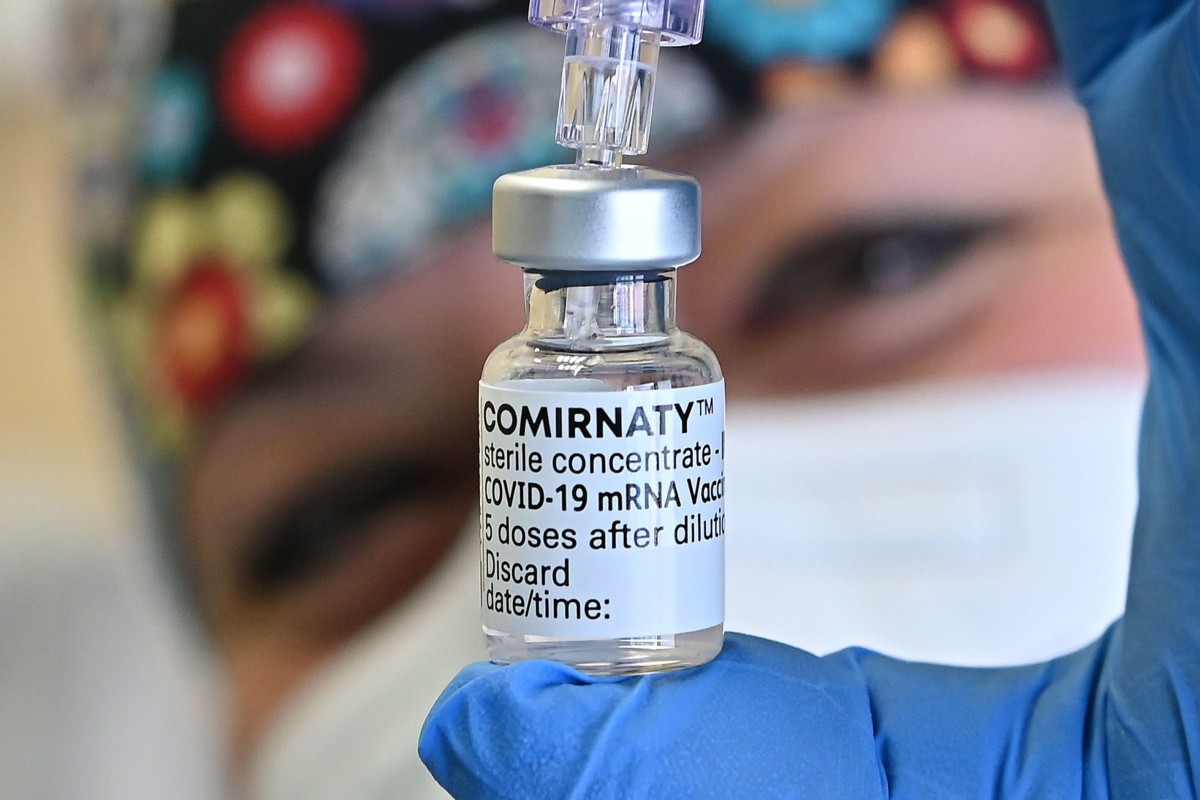 A healthcare worker holds up a vial of the Pfizer-BioNTech Covid-19 vaccine at Molinette hospital in Turin, Italy. The pharmaceutical giant said the vaccine was working with children aged five to 11. Photo: EPA-EFE
A healthcare worker holds up a vial of the Pfizer-BioNTech Covid-19 vaccine at Molinette hospital in Turin, Italy. The pharmaceutical giant said the vaccine was working with children aged five to 11. Photo: EPA-EFEHong Kong should not be too quick to vaccinate children aged five to 11 even though Pfizer and BioNTech have announced their vaccine worked for this age group, infectious disease expert Leung Chi-chiu said on Tuesday.
On Monday, US-based Pfizer and its German partner BioNTech announced they will seek authorisation from the US government to administer their vaccine on children aged five to 11, saying that recent trials had proven it to be effective.
The approval could happen within weeks.
Children of asylum seekers are struggling during Covid
The firms have inoculated 2,268 children within this age group with about one third of the dosage administered for those aged 12 or above. In clinical trials, the vaccines generated an immune response that matched what was previously observed in 16 to 25-year-olds. The safety profile was also comparable to those in the older age group.
Pfizer-BioNTech vaccines are being administered in Hong Kong, but Leung said the city should not rush to administer the jabs to children, especially as there aren’t currently any outbreaks in the city and kids aren’t at great risk.
“In the trial, only (about 2,000 children) received the vaccine. We should wait for at least 10,000 or more children in the US to be vaccinated and observe the response,” Leung told Young Post.
He added that the US Food and Drug Administration (FDA) had yet to approve the idea.
All about the Return2HK and Come2HK schemes
Teenagers have been reported to be at risk of myocarditis and pericarditis – typically temporary conditions involving inflammation of tissues around the heart – after getting two doses of the BioNTech jab, and government health advisers recently started recommending they only get one dose.
“If we cannot prove that the benefits [of vaccinating under 12s] would outweigh the risks, we should not push the practice too quickly,” Leung said.
Pfizer and BioNTech said they planned to share their data with the FDA, the European Medicines Agency and other regulators as soon as possible.
WHO says to wait on Covid booster shots
“We are eager to extend the protection afforded by the vaccine to this younger population, subject to regulatory authorisation, especially as we track the spread of the Delta variant and the substantial threat it poses to children,” Albert Bourla, Pfizer’s chief executive officer, said in a statement.
“Since July, paediatric cases of Covid-19 have risen by about 240 per cent in the US – underscoring the public health need for vaccination. These trial results provide a strong foundation for seeking authorisation of our vaccine for children 5 to 11 years old, and we plan to submit them to the FDA and other regulators with urgency.”
Under Hong Kong’s coronavirus restrictions, a school can apply to resume full-day in-person classes if more than 70 per cent of its students and teachers have been vaccinated.
However, under the city’s current inoculation rules, only those aged 12 and above can be vaccinated. That means that while secondary schools can apply to resume full-day sessions, kindergartens and primary schools cannot do so just yet.
On Monday, education minister Kevin Yeung Yun-hung said that 31 secondary schools have submitted their applications for full-day lessons.
