Advertisement
China approves Pfizer’s Covid-19 pill Paxlovid for emergency use
- Oral pill to be given to adults with mild to moderate Covid-19 with co-morbidities, and high risk of progression to severe disease, drug regulator says
- Pfizer will be required to continue with research work and submit the results of follow-up studies for a general approval
Reading Time:3 minutes
Why you can trust SCMP
77
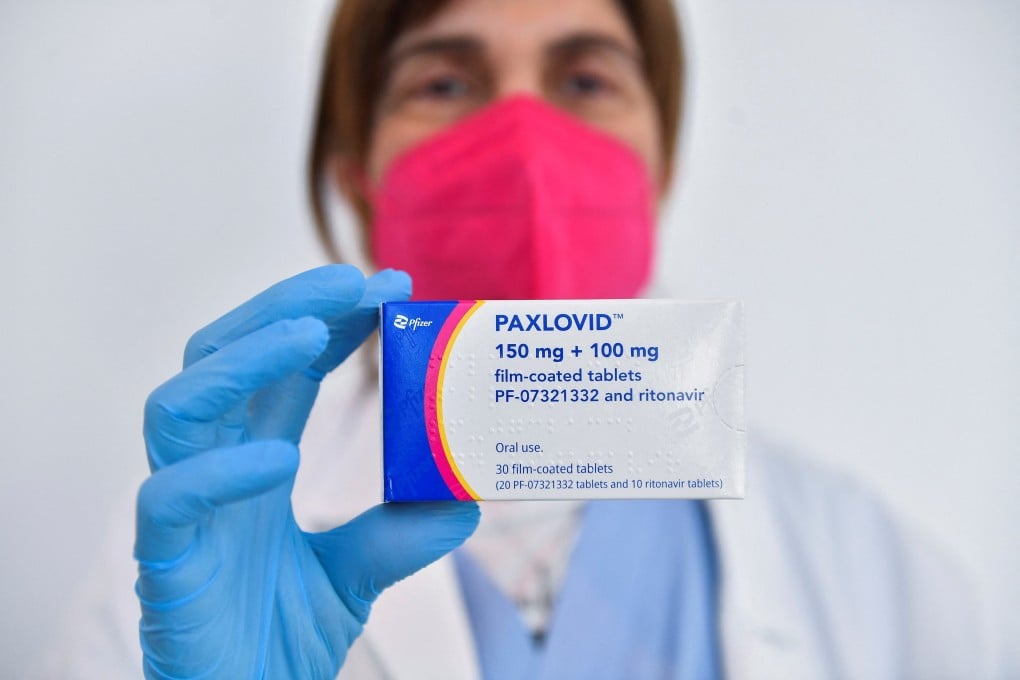
China has approved emergency use of Pfizer’s Covid-19 oral pills – in the country’s first foreign treatment for the coronavirus disease.
Advertisement
The pills, brand named Paxlovid, would be used to treat adults with mild to moderate Covid-19 with high risk of progression to severe disease, the Chinese drug regulator said in announcing the conditional approval on Saturday.
Such patients would include the elderly, and those with chronic kidney issues, cardiovascular or chronic lung disease, diabetes, and other high-risk factors for Covid-19, it said.
“On February 11, the National Medical Products Administration conducted an emergency authorisation review of Paxlovid in accordance with the special drug approval process and an approval with conditions was granted for the import registration of Pfizer’s Covid-19 treatment,” it said in a statement on Saturday morning.
Pfizer will be required to continue with research work and submit the results of follow-up studies for a general approval.
Advertisement

Advertisement
