Japan approves world’s first regenerative medicines using iPS cells
Drugs made with iPS, or induced pluripotent stem cells, will be used to treat patients with severe heart failure and Parkinson’s disease
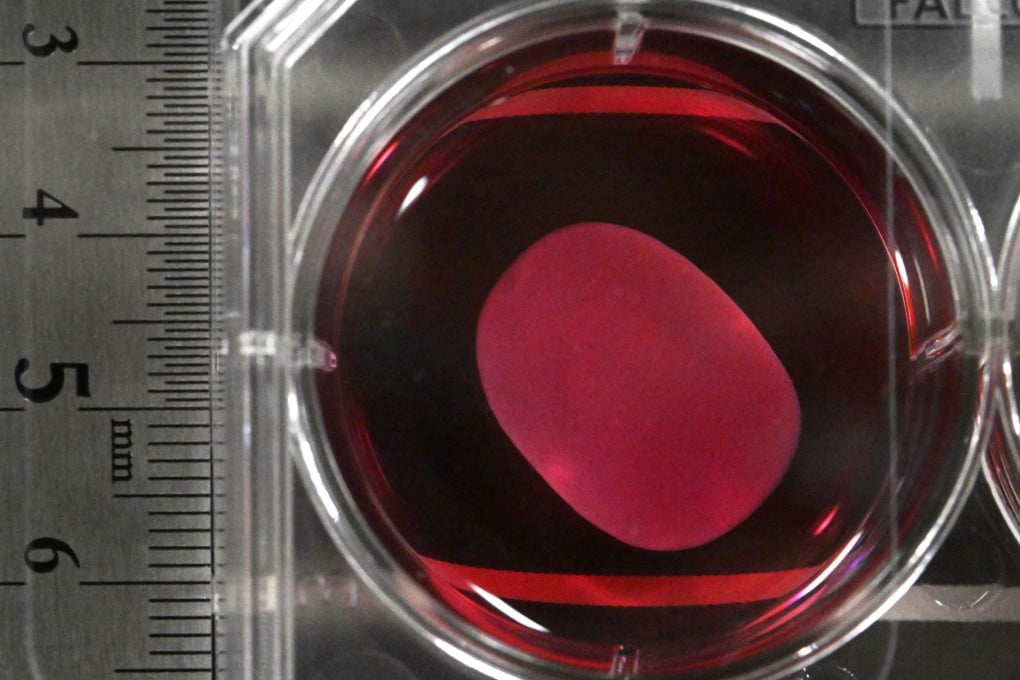
The two drugs, ReHeart developed by Cuorips, a start-up originating from the University of Osaka, and Amchepry by Sumitomo Pharma and Racthera, will be used for patients with severe heart failure stemming from ischemic cardiomyopathy and Parkinson’s disease, respectively.
Induced pluripotent stem cells, or iPS, were generated by Yamanaka, who announced the generation of mouse iPS cells in 2006 and human iPS cells in 2007. He won the Nobel Prize in Physiology or Medicine in 2012.
With ReHeart, heart muscle sheets derived from human iPS cells are placed on the surface of the heart, where they promote the formation of new blood vessels underneath and help restore heart function.
Patients with severe heart disease are often given medicine to prevent deterioration and sometimes may have to undergo a heart transplant.